This section keeps you updated on any major developments throughout the course of the DECISION project. For PDFs of our own press releases please go to DOWNLOADS. To receive our project newsletter please subscribe here!
-
1 March 2024
New review: Acute and non-acute decompensation of liver cirrhosis
-
27 February 2024
Masterclass: Health economic studies in liver diseases by Professor Isabelle Durand-Zaleski
Health economics – a topic quite far from most people’s everyday preoccupations and concerns. But it needn’t be! Discover answers to questions like: What do health economics and shopping for a hoodie have in common? How do you calculate QALYs? Why are drug prices so high and when is a treatment “too expensive”?
Understanding how policy makers utilize health economic studies is not only interesting for healthcare professionals, but also for citizens.
Watch the masterclass on health economic studies in liver diseases by Professor Isabelle Durand-Zaleski, from partner AP-HP:
-
17. January 2024
DECISION project video
We are thrilled to announce the release of our latest video, which dives into the details of the DECISION project – a European research consortium, aiming to enhance our understanding of decompensated cirrhosis and acute-on-chronic liver failure at a systems level. 🎥
Join in, as project partners discuss their roles within the project and emphasise the importancesignificance of collaboration in advancing scientific research. Learn more about the upcoming clinical trial and the role and representation of patients within DECISION.
🔍 Watch the full video on YouTube: https://youtu.be/RelITLtg2w8
-
15 January 2024
Join discussions of DECISION experts
We are thrilled to invite industry stakeholders to be part of our research journey. You now have the opportunity to attend DECISION meetings, where experts of our research program discuss the latest advancements before they reach scientific publications.
By joining these discussions, you gain early access to cutting-edge research in the field of acute decompensation of cirrhosis and ACLF. We believe in collaboration and welcome your involvement.
In exchange for valuable insights, we are open to exploring customized solutions that align with your objectives. Your support will play a crucial role in advancing our research goals.
To secure your spot and discuss tailored opportunities, reach out to us at info[at]decision-for-liver[dot]eu.
Thank you for considering this collaboration. We look forward to welcoming you to our upcoming sessions.

-
12. December 2023
Keynote Lecture: Natural History of Decompensation by Prof. Paolo Angeli
Immerse yourself in the natural history of decompensation with Prof. Dr. Paolo Angeli, Chief of Internal Medicine and Hepatology at University of Padova.
Discover insights into pathophysiology, the EASL-Clif consortium’s new definition of decompensation, and scientific evidence for preventing acute decompensation and ACLF, and much more on acute and non-acute decompensation!
-
24. November 2023
Decompensated Cirrhosis
In 2013, cirrhosis claimed 1.2 million lives globally, with an additional 750,000 liver cancer deaths linked to cirrhosis. Decompensation, marked by severe symptoms, is a critical turning point.
Key Facts:
- Compensated cirrhosis life expectancy: 10-13 years; Decompensation: 2 years.
- Within 1-3 months of decompensation, acute-on-chronic liver failure risk is 11% at day 28, death risk is 5% at day 28, and 14% at day 90.
Treatment Challenges:
- Available treatments include antibiotics, antivirals, diuretics, and more.
- Variable patient response attributed to clinical diversity and overlooked pathophysiological factors.
This clinical heterogeneity calls for novel and personalised combinatorial therapies according to underlying mechanisms based on each patient’s genetics, gender, disease history, and physiology.
-
11. August 2023
DECISION Summer reading
Here are our picks of recent liver research. With contribution by DECISION members:
1️⃣ Assessment of portal hypertension severity using machine learning models in patients with compensated cirrhosis by Jiří Reiniš, Oleksandr Petrenko et al. in Journal of Hepatology
https://www.journal-of-hepatology.eu/article/S0168-8278(22)03119-1/fulltext
2️⃣ Essential role for #albumin in preserving liver cells from TNFα-induced mitochondrial injury by Marta Duran-Güell, Glória Garrabou, Roger Flores-Costa, et al. in FASEB.
https://faseb.onlinelibrary.wiley.com/doi/10.1096/fj.202201526R
3️⃣ Management of splanchnic vein thrombosis by Laure Elkrief, Audrey Payancé, et al. in JHEP Reports.
https://www.jhep-reports.eu/article/S2589-5559(22)00239-7/fulltext
Want more scientific reading? ➡ Check out our publications page!
-
World Hepatitis Day – How DECISION ties in
Each year, 28th July marks World Hepatitis Day – a day to raise awareness of viral hepatitis.
Hepatitis C is a viral infection that leads to liver inflammation, caused by the hepatitis C virus.
According to the World Health Organization (WHO)[1], approximately 15 to 45% of individuals infected with the Hepatitis C virus spontaneously recover within six months of infection without requiring any treatment. Conversely, the remaining 55 to 85% will progress to develop a chronic HCV infection. Within a span of 20 years, around a quarter of those affected by HCV (15 to 30%) will develop cirrhosis, a condition characterized by permanent scarring of the liver.
Cirrhosis can be classified into two stages. The first stage is known as compensated cirrhosis, which indicates that the body is still able to function to some extent despite the presence of reduced liver function and scarring. On the other hand, decompensated cirrhosis represents a more advanced stage where liver functions are deteriorating. In this stage, serious symptoms can arise, such as, jaundice, and kidney failure ascites[2].
DECISION strives to better understand the pathophysiology of decompensated cirrhosis leading to acute-on-chronic liver failure. The ultimate goal is to significantly reduce mortality through combinatorial therapies that are tailored to the specific needs of individual patients.
[1] https://www.who.int/news-room/fact-sheets/detail/hepatitis-c
-
2023-06-24
EASL Clinical Practice Guidelines on acute-on-chronic liver failure published!
We’re especially excited as many DECISION partners are main contributors and the chair is Richard Moreau [INSERM]. New knowledge from DECISION has also been considered.
The objective of the present Clinical Practice Guidelines is to provide recommendations to help clinicians recognise ACLF, make triage decisions (ICU vs. no ICU), identify and manage acute precipitants, identify organ systems that require support or replacement, define potential criteria for futility of intensive care, and identify potential indications for liver transplantation.
View the Clinical Practice Guideline document!
-
Shared networking event at EASL Congress
Join our shared networking and results presentation session with MICROB-PREDICT on Wednesday, 21st June at 13:30 – 14:30 at EASL | The Home of Hepatology’s EASL Congress.
In a shared session, Pierre-Emmanuel Rautou, DECISION coordinator and Jonel Trebicka, MICROB-PREDICT coordinator will moderate flash-talks of the projects’ results, given by DECISION scientists Frank Erhard Uschner, Sara Palomino Echeverría and Estefanía Huergo Iglesias, as well as MICROB-PREDICT researchers Dávid Tornai, Wenyi Gu and Lindsey Ann Edwards.📅Wednesday, 21st June, 2023; 13:30 – 14:30
📍Cirrhosis & Complications Track Hub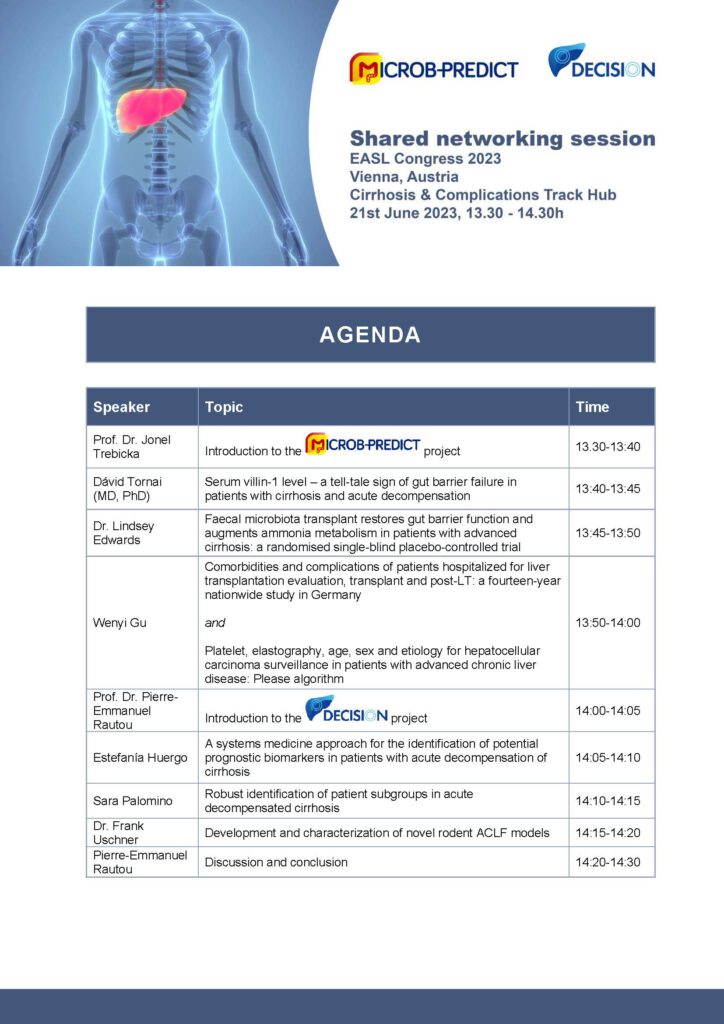
-
12.05.2023
DECISION in EASL Studio
DECISION members Pierre-Emmanuel Rautou and Paolo Caraceni have recently participated in two EASL Studio sessions, a weekly broadcast on news in hepatology and related fields.
Listen to Pierre-Emmanuel Rautou, coordinator of DECISION, in the EASL Studio Season 4, Episode 14 – EASL-EF CLIF and EU Grants: exploring novel mechanism and treatment of chronic liver failure
Additionally, tune in to Paolo Caraceni of UNIBO, contributing to Season 4, Episode 17 – Albumin in Cirrhosis: For All, For Some, For None.
-
17 March 2023
Watch the recording of the patient event on 6th March 2023
The event ‘The COMBAT-trial: A novel COMBinATorial therapy with albumin and enoxaparin in patients with decompensated cirrhosis at high-risk of poor outcome.’ is now available on YouTube.
Tune in as experts from DECISION explain the upcoming clinical trial or discuss the recruitment process.
-
26 January 2023
New Masterclass Video of Agustín Albillos
In this new Masterclass video on YouTube, DECISION consortium member Prof. Dr. Agustín Albillos Martínez (SERMAS) provides an overview of the effects of low molecular weight heparin (LMWH) in cirrhosis, including the mode of action of anti-coagulants, followed by the pathophysiological basis for the long-term use of anticoagulation in cirrhosis as well as the experience with anticoagulants in the clinic. The masterclass took place in the realm of the 4th General Assembly (GA) Meeting on 19th October 2023 in Madrid, Spain, as part of DECISION’s training programme for the project’s early-career scientists (ECS). View all Masterclass videos here.
-
9 December 2022
Study protocol of the COMBAT-TRIAL agreed
After more than one year of intense discussions and exchange on the most promising combination of drugs to be tested in the COMBAT-Trial of DECISION, led by Prof. Paolo Caraceni from Bologna, we are happy to report that the study protocol was finalised. Next steps are the submission to CTIS and regulatory boards and preparation of recruitment. Stay tuned for updates on the trial!
-
25 October 2022
Opening of the 2022/2023 academic year of the Master in Bioethics and Law: Practical lecture on bioethical aspects in biomedical research projects funded by the European Union’s Horizon 2020 programme, taught by Itziar de Lecuona
The opening of the 2022/2023 academic year of the University of Barcelona’s Master in Bioethics and Law will take place on October 25, 2022.
Dr. Itziar de Lecuona, associate professor of the Department of Medicine, director of the Bioethics and Law Observatory and co-director of the Master in Bioethics and Law at the University of Barcelona, will give a practical lecture on bioethical aspects in biomedical research projects funded by the European Union’s Horizon 2020 programme.
This session will be held exclusively for new students of the Master in Bioethics and Law, and for researchers and doctoral students from the Bioethics and Law Observatory.
It will allow the attendees to identify which contributions they could provide from bioethics, based on their profile and knowledge, in the different phases of the project, within the framework of the respective Work Packages on “Ethics, Health and Socioeconomics”. It will intend to train and improve their skills in bioethics in the field of research ethics, scientific integrity and responsible research and innovation.
More information. -
21 October 2022
Free access to EASL educational tools for our early career scientists
We are grateful about a great opportunity for all DECISION early-career scientists (ECS) to benefit from EASL’s extensive educational tools and our Young Investigators (YI) community.EASL campus is currently open access for those individuals with a MyEASL account. In the coming months, some of the content will be restricted to EASL Members. Therefore, EASL invites the project’s ECS to create a MyEASL account and request a complimentary membership. Do not hesitate to reach out to the PMO if you have any questions and more information here.
-
14 October 2022
Publication of the infographic based on the document “Guidelines for reviewing health research and innovation projects that use emergent technologies and personal data”!
The infographic based on the document “Guidelines for reviewing health research and innovation projects that use emergent technologies and personal data”, published by the Bioethics and Law Observatory – UNESCO Chair in Bioethics of the University of Barcelona and coordinated by Dr. Itziar de Lecuona, was presented within the framework of the seminar “Artificial intelligence and data protection in health research and innovation: ethical, legal and social aspects”, on September 21, 2022.
Dr. Itziar de Lecuona, associate professor of the Department of Medicine, director of the Bioethics and Law Observatory and co-director of the Master in Bioethics and Law at the University of Barcelona, showed the infographic, which synthesizes and highlights the essential points developed in the document, during her lecture “Research ethics committees and data protection in the evaluation of emerging technologies in health research and innovation”.
In this way, the infographic exposes, in a visual and concise way, what Research Ethics Committees (RECs) are, their functions, and the dilemmas that health research and innovation projects raise, especially in a scenario where emerging technologies and the exploitation of personal data play a leading role.
The infographic presents the challenges that Research Ethics Committees (RECs) must face (avoid the Europe’s excessive dependence on the American tech companies, update the obsolete protocols for obtaining informed consent, etc.); and lists a series of recommendations so that RECs can perform their functions correctly and efficiently, with the support of both research and innovation centres and legislators, to guarantee the protection of the rights of individuals in projects that use emerging technologies, such as artificial intelligence, big data, biometrics, and/or virtual reality. -
30 September 2022
Checklist for participants to assure informed consent / other mechanisms for those unable to give a written consent
Use our new checklist to assure informed consent for medical research! It facilitates compliance with legal and ethical guidelines related to research involving humans, use of biological samples, and/or personal data. Read more.
-
23 September 2022
Watch our mini-video series from #ILC2022
Following the patients’ interest in DECISION, we tried something new during the International Liver Congress (ILC) in London in June 2022, namely recording several video interviews by representatives of the European Liver Patients’ Association (ELPA) with our scientific coordinator Prof. Dr. Pierre-Emmanuel Rautou. And because we were on a roll, the new concentris team member Dennis Cleff also interviewed Prof. Dr. Sara Montagnese (UNIPD) about the symptoms of decompensated cirrhosis via Zoom shortly thereafter. Watch all videos here.
-
21 September 2022
Bioethics and Law Observatory organises an online seminar
On September 21, 2022, the Bioethics and Law Observatory (OBD) -UNESCO Chair in Bioethics and the Spanish Data Protection Agency (AEPD) jointly organize the seminar “Inteligencia artificial y protección de datos en investigación e innovación en salud: aspectos éticos, legales y sociales”(“Artificial intelligence and data protection in health research and innovation: ethical, legal and social aspects”).
This seminar is part of an agreement between the Bioethics and Law Observatory (OBD) and the Spanish Data Protection Agency.
This event is free and open for anyone, but please note that it will be held in Spanish. For more information click here, find here the program.
-
14 January 2022
Liver seminar about extracellular vesicles in cirrhosis
Watch the latest liver seminar of DECISION coordinator, Prof. Pierre-Emmanuel Rautou, of January, 14th 2022 and learn more about extracellular vesicles in cirrhosis.
-
13 January 2022
Jonel Trebicka’s Masterclass now on YouTube
Watch our newest Masterclass by DECISION consortium member Prof. Dr. Jonel Trebicka who also serves as the coordinator of the topically related Horizon 2020 project MICROB-PREDICT. He provides a step-by-step guide on how to write a high-quality scientific paper. Besides fun examples of what to do and not to do, his advice includes reducing complexity, focussing on key results, displaying the distribution of data points, as well as avoiding lengthy introductions, poetic language, or complicated sentences.
-
14 December 2021
Paolo Angeli’s Masterclass now on YouTube
In this Masterclass, invited speaker Prof. Dr. Paolo Angeli, the current editor-in-chief of the Journal of Hepatology, teaches his approach to write a good scientific paper and submit it to the appropriate journal, a process that he compares to the challenge of winning a soccer game. While focussing on concrete advice, he also points out several common mistakes that less experienced authors make, some of which can be easily avoided. How? Watch the video!
-
29 November 2021
Submission of first periodic report to European Commission
Today, the DECISION Consortium submitted the first periodic report to the European Commission. Over a period of more than two months all partners worked hard to report work performed, results and efforts. The PMO thanks all involved people for their great collaboration and diligent work.
-
03 November 2021
Theresa Wirtz appointed as new chair of the Early Career Scientists group
We congratulate Theresa Wirtz from University of Aachen for having been appointed as new chair of the DECISION Early Career Scientists group. She takes this important position over from her predecessor Frank Uschner (GUF) who was the chair in the first year of the project.
Thanks to Frank for his efforts to coordinate the training activities during the 1st year which included amongst other things the organisation of the 1st masterclass!
-
27 - 30 October 2021
BavenoVII Consensus Workshop sponsored by DECISION
DECISION supports this year`s BavenoVII Consensus Workshop. Baveno Workshops have been held every 5 years from 1990 till today. Each time, important advances have been made in understanding the pathophysiology of portal hypertension, as well as in developing new treatments and new strategies for the management of advanced cirrhosis and portal hypertension. See programme.
-
09 September 2021
DECISION Poster Award
Congratulations to Dr Cristina López-Vicario (Researcher, Fundació Clínic per la Recerca Biomèdica) for winning the first prize of the DECISION Poster Award.
A total of 8 posters were shown during this year’s poster session at the General Assembly Meeting, on 9th September 2021 in Barcelona/Spain.
For further information, click here.
-
19 August 2021
DECISION: Cirrhosis – Overview of existing therapies, drugs & research needs, Pierre-Emmanuel Rautou
Watch video on YouTubeIn this video, the project’s scientific coordinator, Prof. Pierre-Emmanuel Rautou, explains the complications that can occur in cirrhosis patients and how the DECISION project aims to better stratify patients that come to the clinic with acute decompensation (of cirrhosis). The goal is to personalise the treatment of individual patients based on tests that can predict the expected treatment outcome. The video is a recording of the 1st presentation given at an online patient event that was jointly organised by ELPA and EF CLIF and took place on 9th June 2021.
-
09 June 2021
Successful patient event on 9th June
27 representatives of national patient organisations from 10 European countries listened to talks about therapies for cirrhosis and ACLF, research needs and outlook for the future. A lot of interest was shown towards available and new therapies for decompensated cirrhosis and ACLF. The event was organised by ELPA (president Marko Korenjak) and chaired by Prof. Rajiv Jalan, scientific director of EF CLIF.
-
24 June 2021
ILC 2021: Symposium „Old and new treatments for patients with advanced cirrhosis”
Very successful symposium on Thursday, 24th June 2021 from 14-15h CEST organised by members of DECISION in the frame of the virtual ILC congress. Topics included:
Why and how to modulate the microbiome: example from MICROB-PREDICT study (Shawcross)Old drugs for novel treatment strategies (Posé)
Human albumin: from established indications to novel perspectives (Caraceni)
System medicine: is it the right track to personalized treatments: example from DECISION study? (Trebicka)
-
15 April 2021
DECISION Steering Committee Meeting
From 14th – 15th April, the DECISION Steering Committee met again fully virtual. 20 participants exchanged information about progress and results. Great achievement: all ACLARA samples, which are relevant for the work of DECISION, now reached the partner labs. Now the work on these samples can start and we cannot wait to see results.
-
02 March 2021
Pierre-Emmanuel Rautou is being interviewed by “Science et Vie”
In its March issue, the French journal for laymen on science “Science et Vie” interviewed DECISION coordinator Pierre-Emmanuel Rautou on the topic of multi-omics and the acceleration of biomedicine. By decoding the hidden information at the heart of our cells, multi-omics is revolutionising many areas of research. Click below to read further [Articles in French]:
-
25 November 2020
Successful virtual General Assembly Meeting
On 24-25 Nov 2020, 51 members of the DECISION consortium and 4 members of the Scientific and Ethical Advisory Board met online to discuss the progress made during the first 8 months and important next steps. Despite the COVID-19 pandemic, the project is largely on track and important first results have been delivered. Still we all cannot wait to meet in person next time to speed up the communication and international collaboration!
-
10 September 2020
Codes of conduct applicable and research integrity policy including publications in journals published
Read MoreThis document is an analysis of the current codes of good conduct published by each member of the DECISION project: Here, we discuss what is included in the DECISION partners’ code of conduct, any references to national or international guidelines, how easily accessible the relevant information is from the home page and the general usefulness of the document for the members of the institutions themselves and the public.
-
31 August 2020
Public engagement strategy to design DECISION along with the need of patients finalized
Read MoreThe goal of this policy is to provide a framework of public engagement in research in DECISION to align this project with the Responsible Research and Innovation (RRI) framework.
-
27 August 2020
Prof. Dr. Paolo Caraceni from UNIBO, Italy, explains the clinical trial of DECISION
View video on YouTubeIn this video, Prof. Dr. Paolo Caraceni explains the clinical trial, the predictive and response tests, the project’s approach to best-possible patient stratification, and the expected beneficial outcome of DECISION.
-
28 July 2020
DECISION in less than 5 minutes: Prof. Pierre-Emmanuel Rautou explains the project in a brief video
Watch video on YouTubeTake a few minutes to listen to Prof. Pierre-Emmanuel Rautou, the scientific coordinator of DECISION, explain and show to you what the project is all about.
-
27 July 2020
First Patient Event on 31st Aug 2020!
View announcement and programmeSAVE THE DATE: We kindly invite you to the 1st DECISION and ELPA patient event. Our first patient event takes place ONLINE on Monday, August 31st, from 16-17h at Central European Standard Time (CEST). For questions and registration, please contact office@elpa.eu.
-
27 July 2020
Important milestones achieved!
We are happy to report that all ethics approvals for data transfers have been received and 5348 samples from PREDICT and 400 from CANONIC have been sent for analysis to all participating labs. Congratulations WP1 team!
-
22 July 2020
ELPA president Marko Korenjak introduces DECISION from the perspective of cirrhosis patients in this video
Watch video on YouTubeIn this 3-min-long video, Marko Korenjak, the current president of the European Liver Patients Association (ELPA), who is also a key project partner, introduces the scope and aims of the DECISION project.
-
19 July 2020
Interleukin-22 in acute-on-chronic liver failure (ACLF): A matter of ineffective levels, receptor dysregulation, or defective signalling?
Read original articleRead the new article by Schwarzkopf et al. published in J Hep on 19 July 2020. This work addresses one of the new models used in DECISION: The authors analysed the effects of interleukin 22 (IL-22) in a mouse model of ACLF after chronic carbon tetrachloride (CCl4)-treatment (0.2 ml/kg, 2 times per week, i.p.) and acute precipitating injury using 2 injections of 0.4 ml/kg CCl4 in combination with an i.p. challenge with Klebsiella pneumonia, mimicking a bacterial infection.
-
13 July 2020
The PREDICT study uncovers three clinical courses in acutely decompensated cirrhosis with distinct pathophysiology
Read original articleTo access the full article published on 13 July 2020 by Trebicka et al., click on the link below.
-
26 May 2020
The DECISION consortium elects members of the Impact Board
View all our advisory boardsToday, we announce the members of the DECISION Impact Board (IB): Pierre-Emmanuel Rautou, Narsis Kiani, Marko Korenjak, Richard Moreau, Jonel Trebicka, Frank Uschner. The IB is chaired by Christian Trautwein.
-
13 May 2020
Project management talk on YouTube
DECISION’s project manager, Dr. Mary Gazea describes the most critical aspects of the project’s governance structure and the financial issues in a confidential YouTube video. All consortium members are highly encouraged to log into the intranet (KEYWAYS) and watch her presentation. It contains very valuable information to navigate successfully through the coming project years!
-
01 April 2020
DECISION kicks off!
Not an April Fool’s joke – Today, the 5.5-year long, pan-European research project begins. We are very excited to roll up our sleeves and start working!