"Science and everyday life cannot and should not be separated."
In 2013, cirrhosis was responsible for 1.2 million deaths worldwide. This mortality is mainly due to cirrhosis decompensation, i.e. development of ascites, hepatic encephalopathy, and/or gastrointestinal haemorrhage, and its progression to acute-on-chronic liver failure (ACLF). Patients with decompensated cirrhosis receive many treatments such as intravenous and oral absorbable antibiotics, oral non-absorbable antibiotics, albumin, proton-pump inhibitors, laxatives, diuretics, beta-blockers, vasoconstrictors, statins, anticoagulants, steroids, and antiviral agents. Despite these multiple treatments, ACLF or mortality in patients with decompensation of cirrhosis remains high (15% at day 28, and 28% at day 90) because of large interindividual variability in precipitating events, in clinical presentation and in response to treatment. This heterogeneity calls for treatment personalization according to underlying mechanisms.
The objective of DECISION is to enhance our understanding, at systems level, of the pathophysiology of decompensation of cirrhosis leading to ACLF or death to decrease patients’ mortality at day 28.
To translate our science into impact and change in everyday life of cirrhosis patients and clinicians, DECISION will first improve our knowledge of the pathophysiology of decompensated cirrhosis at systems biology level by integrating results of high-throughput multi-omic profiling with comprehensive clinical data from 2,200 fully characterized patients (more than 8,600 time-points) with available standardized biological samples. Second, we will identify novel combinatorial therapies for patients with decompensation of cirrhosis to prevent death. Third, we will refine these therapies in new and/or optimized animal models and then test the best combination in high risk patients in a phase II clinical trial built in DECISION. And fourth, we will develop 2 tests: one predicting outcome of patients with decompensation of cirrhosis when treated with standard treatment (prognostic test); and the other identifying patients who will respond to the novel combinatorial therapy (test for response).
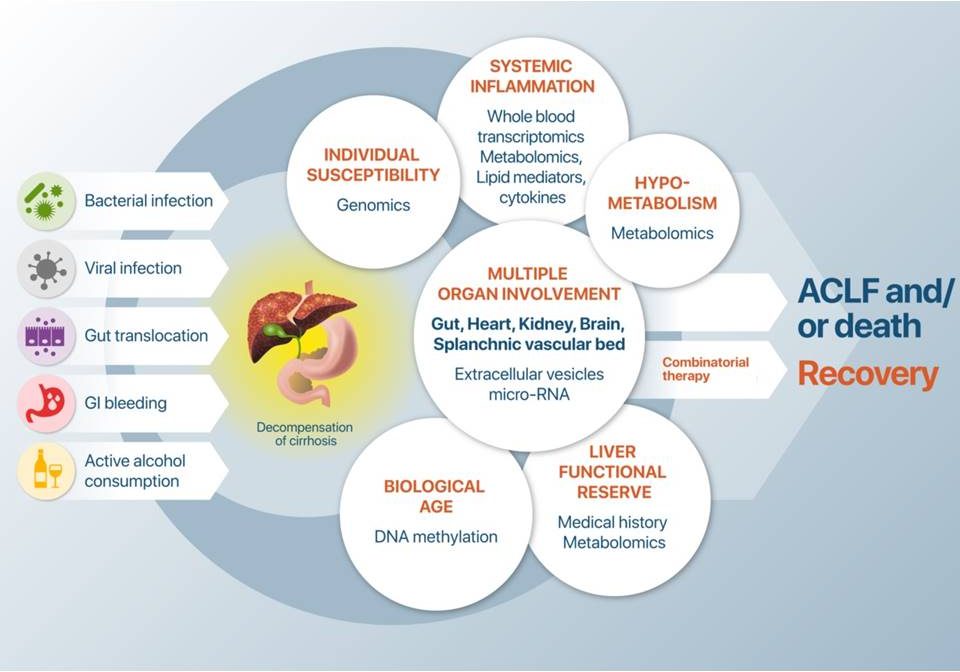
New pathophysiological concepts and molecular profiling of mechanistically similar cases of acute decompensated cirrhosis