"Vision without action is a dream, action without vision is passing time, action with vision is making a difference."
Under the aegis of EF CLIF, DECISION gathers the expertise from 21 different European partners (hospitals, research foundations and institutes, patient and physician associations, and small-and-medium-sized enterprises) to accomplish the following specific objectives:
- To analyse, elucidate, and dissect the pathophysiology of acute decompensation of cirrhosis and its transition to ACLF and death at a systemic level, combining genomics, epigenomics, microRNA, transcriptomics, metabolomics, and inflammatory mediators, as well as extracellular vesicles released by injured organs, with patients’ clinical features and response to treatments, as well as to explore the exact contribution of treatments to the outcome of decompensation of cirrhosis, and thereby to provide important foundations for the development of future combinatorial therapies of available treatments (WPs 1-3).
- To identify new combinatorial therapies, tailored to the needs of specific groups of patients with decompensation of cirrhosis, to prevent ACLF and death (WPs 3-5), refine these therapies in optimised rat models and then test the best combination in a phase II clinical trial built in the DECISION project. This approach focusing on drugs currently used in patients with decompensated cirrhosis minimizes the risk of safety issues. The cost-effectiveness of the new strategy will additionally be assessed with the ultimate aim of decreasing the social and healthcare burden of decompensation of cirrhosis (WP6).
- To refine existing and develop new animal (rat) models of decompensation of cirrhosis leading to ACLF and characterize these models using targeted transcriptomics and metabolomics approaches based on findings obtained in patients (WP1 and WP4).
- To develop novel tests able to predict the treatment outcome of patients with decompensated cirrhosis (prognostic test) and to identify the patient population responding to the developed combinatorial therapy (test for response) (WPs 3-5).
- To disseminate our findings, new combinatorial therapies and tests to the widest possible audience with the help of the European Association for the Study of the Liver (EASL) and the European Liver Patients Association (ELPA) (WP6 and WP7).
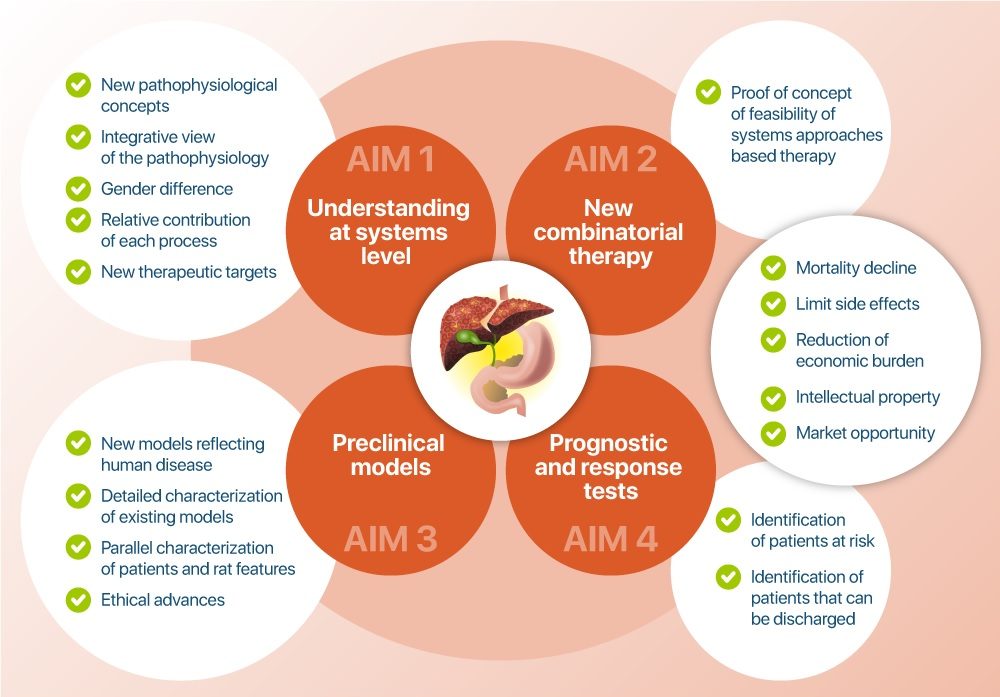
DECISION’s key aims and expected impact